Why Gamma Sterilisation?
Reap benefits of cost and sterility assurance by destroying DNA of micro organisms
What is Gamma Radiation Sterilisation?
Before we explain what Gamma radiation sterilisation is, we should understand what sterilisation itself is and the need for the same.
Sterilisation is a process by which all forms of microbial life are inactivated so that they are not able to reproduce, or such that the probability of their survival is less than one in one million. There are various processes available for bulk sterilisation heat, moist heat, gas (ethylene oxide) and ionising radiation.
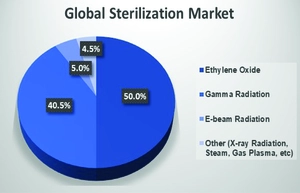
Image Courtesy: Global Sterilization Market Volume Shares, by Sterilization Technology. [4] (Source: E. Goronzy)
Heat Sterilisation
Dry Heat Sterilization
It is carried out by maintaining the product at 160ºC-170ºC for a period not less than two hours. It is suitable for heat resistant products, such as glassware, metal ware and some pharmaceuticals.
Moist Heat Sterilization
It is carried out by air-free, saturated steam under 15 lbs pressure, at not less than 121ºC for a minimum holding time of 15 minutes. This method is suitable for heat stable materials like cotton, gauze, etc.
Chemical/ Irradiation
Ethylene Oxide (ETO)
ETO sterilisation is generally employed for heat and radiation sensitive materials. Ethylene oxide in either pure form or admixed with inert gases is used.
Gamma Irradiation
Gamma rays emanating from artificially produced radioisotopes (such as Cobalt-60 or Caesium-137). The major advantage of radiation is that only one parameter, i.e. the time of irradiation, needs to be controlled. This process is considered far safer compared to ETO.
Gamma Irradiation
Irradiation is a cold process, with a temperature rise of not more than a few degrees centigrade. The process is particularly suitable for industrial scale sterilisation of heat sensitive products, enclosed in air and moisture proof packs in shipping cartons. The radiation dose required for the destruction of fungi and majority of bacteria varies from 1.5 to 15 kGy.
A radiation dose of 25 kGy (2.5 Mrad) is the officially accepted dose for medical product sterilization in many countries. The FDA (USA) and UK panel have accepted the concept of dosimetric release for radiation sterilized medical devices manufactured under GMP. No post sterility microbial testing is required with radiation if dosimetric release procedures are followed.
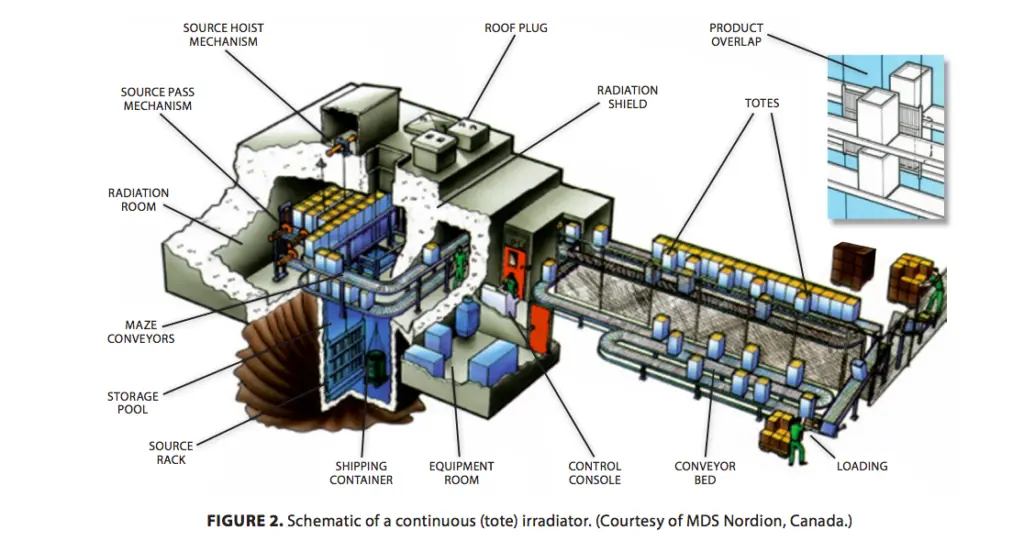
Schematic of an Irradiation Plant
Knowledge Base
— Learn More About Irradiation

